CompDb objects provide access to general (metabolite) compound
annotations along with metadata information such as the annotation's
source, date and release version. The data is stored internally in a
database (usually an SQLite database).
hasMsMsSpectra returns TRUE if MS/MS spectrum data is
available in the database and FALSE otherwise.
Usage
CompDb(x, flags = SQLITE_RO)
hasMsMsSpectra(x)
src_compdb(x)
tables(x)
copyCompDb(x, y)
# S4 method for class 'CompDb'
dbconn(x)
# S4 method for class 'CompDb'
Spectra(object, filter, ...)
# S4 method for class 'CompDb'
supportedFilters(object)
# S4 method for class 'CompDb'
metadata(x, ...)
# S4 method for class 'CompDb'
spectraVariables(object, ...)
# S4 method for class 'CompDb'
compoundVariables(object, includeId = FALSE, ...)
# S4 method for class 'CompDb'
compounds(
object,
columns = compoundVariables(object),
filter,
return.type = c("data.frame", "tibble"),
...
)
# S4 method for class 'CompDb,Spectra'
insertSpectra(object, spectra, columns = spectraVariables(spectra), ...)
# S4 method for class 'CompDb'
deleteSpectra(object, ids = integer(0), ...)
# S4 method for class 'CompDb'
mass2mz(x, adduct = c("[M+H]+"), name = "formula")
# S4 method for class 'CompDb'
insertCompound(object, compounds = data.frame(), addColumns = FALSE)
# S4 method for class 'CompDb'
deleteCompound(object, ids = character(), recursive = FALSE, ...)Arguments
- x
For
CompDb():character(1)with the file name of the SQLite compound database. Alternatively it is possible to provide the connection to the database with parameterx. ForcopyCompDb(): either aCompDbor a database connection.- flags
flags passed to the SQLite database connection. See
RSQLite::SQLite(). Defaults to read-only, i.e.RSQLite::SQLITE_RO.- y
For
copyCompDb(): connection to a database to which the content should be copied.- object
For all methods: a
CompDbobject.- filter
For
compounds()andSpectra(): filter expression orAnnotationFilter::AnnotationFilter()defining a filter to be used to retrieve specific elements from the database.- ...
additional arguments. Currently not used.
- includeId
for
compoundVariables():logical(1)whether the comound ID (column"compound_id") should be included in the result. The default isincludeIds = FALSE.- columns
For
compounds(),Spectra:characterwith the names of the database columns that should be retrieved. UsecompoundVariables()and/orspectraVariables()for a list of available column names. ForinsertSpectra(): columns (spectra variables) that should be inserted into the database (to avoid inserting all variables).- return.type
For
compounds(): either"data.frame"or"tibble"to return the result as adata.frame()ortibble::tibble(), respectively.- spectra
For
insertSpectra():Spectraobject containing the spectra to be added to theIonDbdatabase.- ids
For
deleteSpectra():integer()specifying the IDs of the spectra to delete. IDs inidsthat are not associated to any spectra in theCompDbobject are ignored. FordeleteCompound:character()with the compound IDs to be deleted.- adduct
either a
characterspecifying the name(s) of the adduct(s) for which the m/z should be calculated or adata.framewith the adduct definition. SeeadductNames()for supported adduct names and the description for more information on the expected format if adata.frameis provided.- name
For
mass2mz():character(1). Defines theCompDbcolumn that will be used to name/identify the returned m/z values. By default (name = "formula") m/z values for all unique molecular formulas are calculated and these are used asrownamesfor the returnedmatrix. Withname = "compound_id"the adduct m/z for all compounds (even those with equal formulas) are calculated and returned.- compounds
For
insertCompound():data.framewith compound data to be inserted into aCompDbdatabase. See function description for details.- addColumns
For
insertCompound():logical(1)whether all (extra) columns in parametercompoundsshould be stored also in the database table. The default isaddColumns = FALSE.- recursive
For
deleteCompound():logical(1)whether also MS2 spectra associated with the compounds should be deleted.
Details
CompDb objects should be created using the constructor function
CompDb() providing the name of the (SQLite) database file providing
the compound annotation data.
Retrieve annotations from the database
Annotations/compound informations can be retrieved from a CompDb database
with the compounds() and Spectra() functions:
compounds()extracts compound data from theCompDbobject. In contrast tosrc_compdbit returns the actual data as adata.frame(ifreturn.type = "data.frame") or atibble::tibble()(ifreturn.type = "tibble"). Acompounds()call will always return all elements from the ms_compound table (unless afilteris used).Spectra()extract spectra from the database and returns them as aSpectra::Spectra()object from the Spectra package. Additional annotations requested with thecolumnsparameter are added as additional spectra variables.
General functions
CompDb(): connect to a compound database.compoundVariables(): returns all available columns/database fields for compounds.copyCompDb(): allows to copy the content from a CompDb to another database. Parameterxis supposed to be either aCompDbor a database connection from which the data should be copied andya connection to a database to which it should be copied.dbconn(): returns the connection (of typeDBIConnection) to the database.metadata(): returns general meta data of the compound database.spectraVariables(): returns all spectra variables (i.e. columns) available in theCompDb.src_compdb()provides access to theCompDb's database via the functionality from thedplyr/dbplyrpackage.supportedFilters(): provides an overview of the filters that can be applied on aCompDbobject to extract only specific data from the database.tables(): returns a namedlist(names being table names) with the fields/columns from each table in the database.mass2mz(): calculates a table of the m/z values for each compound based on the provided set of adduct(s). Adduct definitions can be provided with parameteradduct. SeeMetaboCoreUtils::mass2mz()for more details. Parameternamedefines the database table column that should be used asrownamesof the returnedmatrix. By defaultname = "formula", m/z values are calculated for each unique formula in theCompDbx.
Adding and removing data from a database
Note that inserting and deleting data requires read-write access to the
database. Databases returned by CompDb are by default read-only. To get
write access CompDb should be called with parameter
flags = RSQLite::SQLITE_RW.
insertCompound(): adds additional compound(s) to aCompDb. The compound(s) to be added can be specified with parametercompoundsthat is expected to be adata.framewith columns"compound_id","name","inchi","inchikey","formula","exactmass". Column"exactmass"is expected to contain numeric values, all other columnscharacter. Missing values are allowed for all columns except"compound_id". An optional column"synonyms"can be used to provide alternative names for the compound. This column can contain a singlecharacterby row, or alistwith multiplecharacter(names) per row/compound (see examples below for details). By setting parameteraddColumns = TRUEany additional columns incompoundwill be added to the database table. The default isaddColumns = FALSE. The function returns theCompDbwith the compounds added. See alsocreateCompDb()for more information and details on expected compound data and the examples below for general usage.deleteCompound(): removes specified compounds from theCompDbdatabase. The IDs of the compounds that should be deleted need to be provided with parameterids. To include compound IDs in the output of acompounds()call"compound_id"should be added to thecolumnsparameter. By default an error is thrown if for some of the specified compounds also MS2 spectra are present in the database. To force deletion of the compounds along with all associated MS2 spectra userecursive = TRUE. See examples below for details. The function returns the updatedCompDbdatabase.insertSpectra(): adds further spectra to the database. The method always adds all the spectra specified through thespectraparameter and does not check if they are already in the database. Note that the input spectra must have the variablecompound_idand onlySpectrawhosecompound_idvalues are also incompounds(object, "compound_id")can be added. Parametercolumnsdefines which spectra variables from thespectrashould be inserted into the database. By default, all spectra variables are added but it is strongly suggested to specifically select (meaningful) spectra variables that should be stored in the database. Note that a spectra variable"compound_id"is mandatory. If needed, the function adds additional columns to themsms_spectrumdatabase table. The function returns the updatedCompDbobject.deleteSpectra(): deletes specified spectra from the database. The IDs of the spectra to be deleted need to be provided with parameterids.
Note that it is also possible to add new database tables and include them in
the data retrieval queries. See addJoinDefinition() for more information.
Filtering the database
Data access methods such as compounds() and Spectra allow to filter the
results using specific filter classes and expressions. Filtering uses the
concepts from Bioconductor's AnnotationFilter package. All information
for a certain compound with the ID "HMDB0000001" can for example be
retrieved by passing the filter expression
filter = ~ compound_id == "HMDB0000001" to the compounds function.
Use the AnnotationFilter::supportedFilters() function on the CompDb
object to get a list of all supported filters. See also examples below
or the usage vignette for details.
See also
createCompDb()for the function to create a SQLite compound database.CompoundIdFilter()for filters that can be used on theCompDbdatabase.addJoinDefinition()to expand a CompDb with additional, related, database tables.
Examples
## We load a small compound test database based on MassBank which is
## distributed with this package.
cdb <- CompDb(system.file("sql/CompDb.MassBank.sql", package = "CompoundDb"))
cdb
#> class: CompDb
#> data source: MassBank
#> version: 2020.09
#> organism: NA
#> compound count: 70
#> MS/MS spectra count: 70
## Get general metadata information from the database, such as originating
## source and version:
metadata(cdb)
#> name value
#> 1 source MassBank
#> 2 url https://massbank.eu/MassBank/
#> 3 source_version 2020.09
#> 4 source_date 1603272565
#> 5 organism <NA>
#> 6 db_creation_date Thu Oct 22 08:45:31 2020
#> 7 supporting_package CompoundDb
#> 8 supporting_object CompDb
## List all available compound annotations/fields
compoundVariables(cdb)
#> [1] "formula" "exactmass" "smiles" "inchi" "inchikey" "cas"
#> [7] "pubchem" "name"
## Extract a data.frame with these annotations for all compounds
compounds(cdb)
#> formula exactmass
#> 1 C10H10O3 178.0630
#> 2 C25H47NO9 505.3251
#> 3 C17H12O6 312.0634
#> 4 C17H14O6 314.0790
#> 5 C17H12O7 328.0583
#> 6 C17H14O7 330.0739
#> 7 C17H12O7 328.0583
#> 8 C20H20N2O3 336.1474
#> 9 C15H16O6 292.0947
#> 10 C14H10O5 258.0528
#> 11 C15H12O5 272.0685
#> 12 C16H16O8 336.0845
#> smiles
#> 1 CC1CC2=C(C(=CC=C2)O)C(=O)O1
#> 2 CC[C@@H](C)[C@H]([C@H](C[C@@H](C)CCCCCC[C@H](C[C@@H](CN)O)O)OC(=O)CC(CC(=O)O)C(=O)O)O
#> 3 COC1=C2C3=C(C(=O)CC3)C(=O)OC2=C4[C@@H]5C=CO[C@@H]5OC4=C1
#> 4 COC1=C2C3=C(C(=O)CC3)C(=O)OC2=C4[C@@H]5CCO[C@@H]5OC4=C1
#> 5 COC1=C2C3=C(C(=O)OCC3)C(=O)OC2=C4[C@@H]5C=CO[C@@H]5OC4=C1
#> 6 COC1=C2C3=C(C(=O)OCC3)C(=O)OC2=C4[C@@H]5CCO[C@@H]5OC4=C1
#> 7 COC1=C2C3=C(C(=O)CC3)C(=O)OC2=C4C(=C1)O[C@@H]5[C@]4(C=CO5)O
#> 8 CC(=O)C1=C([C@@H]2[C@@H]3[C@@H](CC4=C5C3=CNC5=CC=C4)C(N2C1=O)(C)C)O
#> 9 C[C@]12C[C@@H]([C@H](C=C1C3=CC(=CC(=C3C(=O)O2)O)OC)O)O
#> 10 CC1=CC(=CC2=C1C3=CC(=CC(=C3C(=O)O2)O)O)O
#> 11 CC1=CC(=CC2=C1C3=CC(=CC(=C3C(=O)O2)O)OC)O
#> 12 C[C@]1([C@@H]([C@H](C2=C([C@H]1O)C(=O)C3=CC(=CC(=C3C2=O)O)OC)O)O)O
#> inchi
#> 1 InChI=1S/C10H10O3/c1-6-5-7-3-2-4-8(11)9(7)10(12)13-6/h2-4,6,11H,5H2,1H3
#> 2 InChI=1S/C25H47NO9/c1-4-17(3)24(32)21(35-23(31)13-18(25(33)34)12-22(29)30)11-16(2)9-7-5-6-8-10-19(27)14-20(28)15-26/h16-21,24,27-28,32H,4-15,26H2,1-3H3,(H,29,30)(H,33,34)/t16-,17+,18?,19+,20-,21-,24+/m0/s1
#> 3 InChI=1S/C17H12O6/c1-20-10-6-11-14(8-4-5-21-17(8)22-11)15-13(10)7-2-3-9(18)12(7)16(19)23-15/h4-6,8,17H,2-3H2,1H3/t8-,17+/m0/s1
#> 4 InChI=1S/C17H14O6/c1-20-10-6-11-14(8-4-5-21-17(8)22-11)15-13(10)7-2-3-9(18)12(7)16(19)23-15/h6,8,17H,2-5H2,1H3/t8-,17+/m0/s1
#> 5 InChI=1S/C17H12O7/c1-20-9-6-10-12(8-3-5-22-17(8)23-10)14-11(9)7-2-4-21-15(18)13(7)16(19)24-14/h3,5-6,8,17H,2,4H2,1H3/t8-,17+/m0/s1
#> 6 InChI=1S/C17H14O7/c1-20-9-6-10-12(8-3-5-22-17(8)23-10)14-11(9)7-2-4-21-15(18)13(7)16(19)24-14/h6,8,17H,2-5H2,1H3/t8-,17+/m0/s1
#> 7 InChI=1S/C17H12O7/c1-21-9-6-10-13(17(20)4-5-22-16(17)23-10)14-12(9)7-2-3-8(18)11(7)15(19)24-14/h4-6,16,20H,2-3H2,1H3/t16-,17-/m1/s1
#> 8 InChI=1S/C20H20N2O3/c1-9(23)14-18(24)17-16-11-8-21-13-6-4-5-10(15(11)13)7-12(16)20(2,3)22(17)19(14)25/h4-6,8,12,16-17,21,24H,7H2,1-3H3/t12-,16+,17+/m1/s1
#> 9 InChI=1S/C15H16O6/c1-15-6-12(18)10(16)5-9(15)8-3-7(20-2)4-11(17)13(8)14(19)21-15/h3-5,10,12,16-18H,6H2,1-2H3/t10-,12-,15-/m0/s1
#> 10 InChI=1S/C14H10O5/c1-6-2-7(15)5-11-12(6)9-3-8(16)4-10(17)13(9)14(18)19-11/h2-5,15-17H,1H3
#> 11 InChI=1S/C15H12O5/c1-7-3-8(16)4-12-13(7)10-5-9(19-2)6-11(17)14(10)15(18)20-12/h3-6,16-17H,1-2H3
#> 12 InChI=1S/C16H16O8/c1-16(23)14(21)10-9(13(20)15(16)22)12(19)8-6(11(10)18)3-5(24-2)4-7(8)17/h3-4,13-15,17,20-23H,1-2H3/t13-,14+,15+,16-/m0/s1
#> inchikey cas pubchem
#> 1 KWILGNNWGSNMPA-UHFFFAOYSA-N 17397-85-2 CID:28516
#> 2 CTXQVLLVFBNZKL-YVEDVMJTSA-N 149849-90-1 CID:102004382
#> 3 OQIQSTLJSLGHID-WNWIJWBNSA-N 1162-65-8 CID:186907
#> 4 WWSYXEZEXMQWHT-WNWIJWBNSA-N 7220-81-7 CID:2724360
#> 5 XWIYFDMXXLINPU-WNWIJWBNSA-N 1165-39-5 CID:2724361
#> 6 WPCVRWVBBXIRMA-WNWIJWBNSA-N 7241-98-7 CID:2724362
#> 7 MJBWDEQAUQTVKK-IAGOWNOFSA-N 6795-23-9 CID:15558498
#> 8 SZINUGQCTHLQAZ-DQYPLSBCSA-N 18172-33-3 CID:54682463
#> 9 MMHTXEATDNFMMY-WBIUFABUSA-N 29752-43-0 CID:34687
#> 10 CEBXXEKPIIDJHL-UHFFFAOYSA-N 641-38-3 CID:5359485
#> 11 LCSDQFNUYFTXMT-UHFFFAOYSA-N 23452-05-3 CID:5360741
#> 12 VSMBLBOUQJNJIL-JJXSEGSLSA-N 22268-16-2 CID:89644
#> name
#> 1 Mellein
#> 2 AAL toxin TB
#> 3 Aflatoxin B1
#> 4 Aflatoxin B2
#> 5 Aflatoxin G1
#> 6 Aflatoxin G2
#> 7 Aflatoxin M1
#> 8 alpha-Cyclopiazonic acid
#> 9 Altenuene
#> 10 Alternariol
#> 11 Alternariol methyl ether
#> 12 Altersolanol A
## Note that the `compounds` function will by default always return a
## data frame of **unique** entries for the specified columns. Including
## also the `"compound_id"` to the requested columns will ensure that all
## data is returned from the tables.
compounds(cdb, columns = c("compound_id", compoundVariables(cdb)))
#> compound_id formula exactmass
#> 1 1 C10H10O3 178.0630
#> 2 2 C10H10O3 178.0630
#> 3 3 C10H10O3 178.0630
#> 4 4 C10H10O3 178.0630
#> 5 5 C10H10O3 178.0630
#> 6 6 C25H47NO9 505.3251
#> 7 7 C25H47NO9 505.3251
#> 8 8 C25H47NO9 505.3251
#> 9 9 C25H47NO9 505.3251
#> 10 10 C25H47NO9 505.3251
#> 11 11 C25H47NO9 505.3251
#> 12 12 C25H47NO9 505.3251
#> 13 13 C25H47NO9 505.3251
#> 14 14 C25H47NO9 505.3251
#> 15 15 C25H47NO9 505.3251
#> 16 16 C17H12O6 312.0634
#> 17 17 C17H12O6 312.0634
#> 18 18 C17H12O6 312.0634
#> 19 19 C17H12O6 312.0634
#> 20 20 C17H12O6 312.0634
#> 21 21 C17H12O6 312.0634
#> 22 22 C17H12O6 312.0634
#> 23 23 C17H12O6 312.0634
#> 24 24 C17H12O6 312.0634
#> 25 25 C17H12O6 312.0634
#> 26 26 C17H14O6 314.0790
#> 27 27 C17H14O6 314.0790
#> 28 28 C17H14O6 314.0790
#> 29 29 C17H14O6 314.0790
#> 30 30 C17H14O6 314.0790
#> 31 31 C17H14O6 314.0790
#> 32 32 C17H12O7 328.0583
#> 33 33 C17H12O7 328.0583
#> 34 34 C17H12O7 328.0583
#> 35 35 C17H12O7 328.0583
#> 36 36 C17H12O7 328.0583
#> 37 37 C17H12O7 328.0583
#> 38 38 C17H14O7 330.0739
#> 39 39 C17H14O7 330.0739
#> 40 40 C17H14O7 330.0739
#> 41 41 C17H14O7 330.0739
#> 42 42 C17H14O7 330.0739
#> 43 43 C17H14O7 330.0739
#> 44 44 C17H12O7 328.0583
#> 45 45 C17H12O7 328.0583
#> 46 46 C17H12O7 328.0583
#> 47 47 C17H12O7 328.0583
#> 48 48 C17H12O7 328.0583
#> 49 49 C17H12O7 328.0583
#> 50 50 C20H20N2O3 336.1474
#> 51 51 C20H20N2O3 336.1474
#> 52 52 C20H20N2O3 336.1474
#> 53 53 C20H20N2O3 336.1474
#> 54 54 C20H20N2O3 336.1474
#> 55 55 C15H16O6 292.0947
#> 56 56 C15H16O6 292.0947
#> 57 57 C15H16O6 292.0947
#> 58 58 C15H16O6 292.0947
#> 59 59 C15H16O6 292.0947
#> 60 60 C14H10O5 258.0528
#> 61 61 C14H10O5 258.0528
#> 62 62 C14H10O5 258.0528
#> 63 63 C15H12O5 272.0685
#> 64 64 C15H12O5 272.0685
#> 65 65 C15H12O5 272.0685
#> 66 66 C16H16O8 336.0845
#> 67 67 C16H16O8 336.0845
#> 68 68 C16H16O8 336.0845
#> 69 69 C16H16O8 336.0845
#> 70 70 C16H16O8 336.0845
#> smiles
#> 1 CC1CC2=C(C(=CC=C2)O)C(=O)O1
#> 2 CC1CC2=C(C(=CC=C2)O)C(=O)O1
#> 3 CC1CC2=C(C(=CC=C2)O)C(=O)O1
#> 4 CC1CC2=C(C(=CC=C2)O)C(=O)O1
#> 5 CC1CC2=C(C(=CC=C2)O)C(=O)O1
#> 6 CC[C@@H](C)[C@H]([C@H](C[C@@H](C)CCCCCC[C@H](C[C@@H](CN)O)O)OC(=O)CC(CC(=O)O)C(=O)O)O
#> 7 CC[C@@H](C)[C@H]([C@H](C[C@@H](C)CCCCCC[C@H](C[C@@H](CN)O)O)OC(=O)CC(CC(=O)O)C(=O)O)O
#> 8 CC[C@@H](C)[C@H]([C@H](C[C@@H](C)CCCCCC[C@H](C[C@@H](CN)O)O)OC(=O)CC(CC(=O)O)C(=O)O)O
#> 9 CC[C@@H](C)[C@H]([C@H](C[C@@H](C)CCCCCC[C@H](C[C@@H](CN)O)O)OC(=O)CC(CC(=O)O)C(=O)O)O
#> 10 CC[C@@H](C)[C@H]([C@H](C[C@@H](C)CCCCCC[C@H](C[C@@H](CN)O)O)OC(=O)CC(CC(=O)O)C(=O)O)O
#> 11 CC[C@@H](C)[C@H]([C@H](C[C@@H](C)CCCCCC[C@H](C[C@@H](CN)O)O)OC(=O)CC(CC(=O)O)C(=O)O)O
#> 12 CC[C@@H](C)[C@H]([C@H](C[C@@H](C)CCCCCC[C@H](C[C@@H](CN)O)O)OC(=O)CC(CC(=O)O)C(=O)O)O
#> 13 CC[C@@H](C)[C@H]([C@H](C[C@@H](C)CCCCCC[C@H](C[C@@H](CN)O)O)OC(=O)CC(CC(=O)O)C(=O)O)O
#> 14 CC[C@@H](C)[C@H]([C@H](C[C@@H](C)CCCCCC[C@H](C[C@@H](CN)O)O)OC(=O)CC(CC(=O)O)C(=O)O)O
#> 15 CC[C@@H](C)[C@H]([C@H](C[C@@H](C)CCCCCC[C@H](C[C@@H](CN)O)O)OC(=O)CC(CC(=O)O)C(=O)O)O
#> 16 COC1=C2C3=C(C(=O)CC3)C(=O)OC2=C4[C@@H]5C=CO[C@@H]5OC4=C1
#> 17 COC1=C2C3=C(C(=O)CC3)C(=O)OC2=C4[C@@H]5C=CO[C@@H]5OC4=C1
#> 18 COC1=C2C3=C(C(=O)CC3)C(=O)OC2=C4[C@@H]5C=CO[C@@H]5OC4=C1
#> 19 COC1=C2C3=C(C(=O)CC3)C(=O)OC2=C4[C@@H]5C=CO[C@@H]5OC4=C1
#> 20 COC1=C2C3=C(C(=O)CC3)C(=O)OC2=C4[C@@H]5C=CO[C@@H]5OC4=C1
#> 21 COC1=C2C3=C(C(=O)CC3)C(=O)OC2=C4[C@@H]5C=CO[C@@H]5OC4=C1
#> 22 COC1=C2C3=C(C(=O)CC3)C(=O)OC2=C4[C@@H]5C=CO[C@@H]5OC4=C1
#> 23 COC1=C2C3=C(C(=O)CC3)C(=O)OC2=C4[C@@H]5C=CO[C@@H]5OC4=C1
#> 24 COC1=C2C3=C(C(=O)CC3)C(=O)OC2=C4[C@@H]5C=CO[C@@H]5OC4=C1
#> 25 COC1=C2C3=C(C(=O)CC3)C(=O)OC2=C4[C@@H]5C=CO[C@@H]5OC4=C1
#> 26 COC1=C2C3=C(C(=O)CC3)C(=O)OC2=C4[C@@H]5CCO[C@@H]5OC4=C1
#> 27 COC1=C2C3=C(C(=O)CC3)C(=O)OC2=C4[C@@H]5CCO[C@@H]5OC4=C1
#> 28 COC1=C2C3=C(C(=O)CC3)C(=O)OC2=C4[C@@H]5CCO[C@@H]5OC4=C1
#> 29 COC1=C2C3=C(C(=O)CC3)C(=O)OC2=C4[C@@H]5CCO[C@@H]5OC4=C1
#> 30 COC1=C2C3=C(C(=O)CC3)C(=O)OC2=C4[C@@H]5CCO[C@@H]5OC4=C1
#> 31 COC1=C2C3=C(C(=O)CC3)C(=O)OC2=C4[C@@H]5CCO[C@@H]5OC4=C1
#> 32 COC1=C2C3=C(C(=O)OCC3)C(=O)OC2=C4[C@@H]5C=CO[C@@H]5OC4=C1
#> 33 COC1=C2C3=C(C(=O)OCC3)C(=O)OC2=C4[C@@H]5C=CO[C@@H]5OC4=C1
#> 34 COC1=C2C3=C(C(=O)OCC3)C(=O)OC2=C4[C@@H]5C=CO[C@@H]5OC4=C1
#> 35 COC1=C2C3=C(C(=O)OCC3)C(=O)OC2=C4[C@@H]5C=CO[C@@H]5OC4=C1
#> 36 COC1=C2C3=C(C(=O)OCC3)C(=O)OC2=C4[C@@H]5C=CO[C@@H]5OC4=C1
#> 37 COC1=C2C3=C(C(=O)OCC3)C(=O)OC2=C4[C@@H]5C=CO[C@@H]5OC4=C1
#> 38 COC1=C2C3=C(C(=O)OCC3)C(=O)OC2=C4[C@@H]5CCO[C@@H]5OC4=C1
#> 39 COC1=C2C3=C(C(=O)OCC3)C(=O)OC2=C4[C@@H]5CCO[C@@H]5OC4=C1
#> 40 COC1=C2C3=C(C(=O)OCC3)C(=O)OC2=C4[C@@H]5CCO[C@@H]5OC4=C1
#> 41 COC1=C2C3=C(C(=O)OCC3)C(=O)OC2=C4[C@@H]5CCO[C@@H]5OC4=C1
#> 42 COC1=C2C3=C(C(=O)OCC3)C(=O)OC2=C4[C@@H]5CCO[C@@H]5OC4=C1
#> 43 COC1=C2C3=C(C(=O)OCC3)C(=O)OC2=C4[C@@H]5CCO[C@@H]5OC4=C1
#> 44 COC1=C2C3=C(C(=O)CC3)C(=O)OC2=C4C(=C1)O[C@@H]5[C@]4(C=CO5)O
#> 45 COC1=C2C3=C(C(=O)CC3)C(=O)OC2=C4C(=C1)O[C@@H]5[C@]4(C=CO5)O
#> 46 COC1=C2C3=C(C(=O)CC3)C(=O)OC2=C4C(=C1)O[C@@H]5[C@]4(C=CO5)O
#> 47 COC1=C2C3=C(C(=O)CC3)C(=O)OC2=C4C(=C1)O[C@@H]5[C@]4(C=CO5)O
#> 48 COC1=C2C3=C(C(=O)CC3)C(=O)OC2=C4C(=C1)O[C@@H]5[C@]4(C=CO5)O
#> 49 COC1=C2C3=C(C(=O)CC3)C(=O)OC2=C4C(=C1)O[C@@H]5[C@]4(C=CO5)O
#> 50 CC(=O)C1=C([C@@H]2[C@@H]3[C@@H](CC4=C5C3=CNC5=CC=C4)C(N2C1=O)(C)C)O
#> 51 CC(=O)C1=C([C@@H]2[C@@H]3[C@@H](CC4=C5C3=CNC5=CC=C4)C(N2C1=O)(C)C)O
#> 52 CC(=O)C1=C([C@@H]2[C@@H]3[C@@H](CC4=C5C3=CNC5=CC=C4)C(N2C1=O)(C)C)O
#> 53 CC(=O)C1=C([C@@H]2[C@@H]3[C@@H](CC4=C5C3=CNC5=CC=C4)C(N2C1=O)(C)C)O
#> 54 CC(=O)C1=C([C@@H]2[C@@H]3[C@@H](CC4=C5C3=CNC5=CC=C4)C(N2C1=O)(C)C)O
#> 55 C[C@]12C[C@@H]([C@H](C=C1C3=CC(=CC(=C3C(=O)O2)O)OC)O)O
#> 56 C[C@]12C[C@@H]([C@H](C=C1C3=CC(=CC(=C3C(=O)O2)O)OC)O)O
#> 57 C[C@]12C[C@@H]([C@H](C=C1C3=CC(=CC(=C3C(=O)O2)O)OC)O)O
#> 58 C[C@]12C[C@@H]([C@H](C=C1C3=CC(=CC(=C3C(=O)O2)O)OC)O)O
#> 59 C[C@]12C[C@@H]([C@H](C=C1C3=CC(=CC(=C3C(=O)O2)O)OC)O)O
#> 60 CC1=CC(=CC2=C1C3=CC(=CC(=C3C(=O)O2)O)O)O
#> 61 CC1=CC(=CC2=C1C3=CC(=CC(=C3C(=O)O2)O)O)O
#> 62 CC1=CC(=CC2=C1C3=CC(=CC(=C3C(=O)O2)O)O)O
#> 63 CC1=CC(=CC2=C1C3=CC(=CC(=C3C(=O)O2)O)OC)O
#> 64 CC1=CC(=CC2=C1C3=CC(=CC(=C3C(=O)O2)O)OC)O
#> 65 CC1=CC(=CC2=C1C3=CC(=CC(=C3C(=O)O2)O)OC)O
#> 66 C[C@]1([C@@H]([C@H](C2=C([C@H]1O)C(=O)C3=CC(=CC(=C3C2=O)O)OC)O)O)O
#> 67 C[C@]1([C@@H]([C@H](C2=C([C@H]1O)C(=O)C3=CC(=CC(=C3C2=O)O)OC)O)O)O
#> 68 C[C@]1([C@@H]([C@H](C2=C([C@H]1O)C(=O)C3=CC(=CC(=C3C2=O)O)OC)O)O)O
#> 69 C[C@]1([C@@H]([C@H](C2=C([C@H]1O)C(=O)C3=CC(=CC(=C3C2=O)O)OC)O)O)O
#> 70 C[C@]1([C@@H]([C@H](C2=C([C@H]1O)C(=O)C3=CC(=CC(=C3C2=O)O)OC)O)O)O
#> inchi
#> 1 InChI=1S/C10H10O3/c1-6-5-7-3-2-4-8(11)9(7)10(12)13-6/h2-4,6,11H,5H2,1H3
#> 2 InChI=1S/C10H10O3/c1-6-5-7-3-2-4-8(11)9(7)10(12)13-6/h2-4,6,11H,5H2,1H3
#> 3 InChI=1S/C10H10O3/c1-6-5-7-3-2-4-8(11)9(7)10(12)13-6/h2-4,6,11H,5H2,1H3
#> 4 InChI=1S/C10H10O3/c1-6-5-7-3-2-4-8(11)9(7)10(12)13-6/h2-4,6,11H,5H2,1H3
#> 5 InChI=1S/C10H10O3/c1-6-5-7-3-2-4-8(11)9(7)10(12)13-6/h2-4,6,11H,5H2,1H3
#> 6 InChI=1S/C25H47NO9/c1-4-17(3)24(32)21(35-23(31)13-18(25(33)34)12-22(29)30)11-16(2)9-7-5-6-8-10-19(27)14-20(28)15-26/h16-21,24,27-28,32H,4-15,26H2,1-3H3,(H,29,30)(H,33,34)/t16-,17+,18?,19+,20-,21-,24+/m0/s1
#> 7 InChI=1S/C25H47NO9/c1-4-17(3)24(32)21(35-23(31)13-18(25(33)34)12-22(29)30)11-16(2)9-7-5-6-8-10-19(27)14-20(28)15-26/h16-21,24,27-28,32H,4-15,26H2,1-3H3,(H,29,30)(H,33,34)/t16-,17+,18?,19+,20-,21-,24+/m0/s1
#> 8 InChI=1S/C25H47NO9/c1-4-17(3)24(32)21(35-23(31)13-18(25(33)34)12-22(29)30)11-16(2)9-7-5-6-8-10-19(27)14-20(28)15-26/h16-21,24,27-28,32H,4-15,26H2,1-3H3,(H,29,30)(H,33,34)/t16-,17+,18?,19+,20-,21-,24+/m0/s1
#> 9 InChI=1S/C25H47NO9/c1-4-17(3)24(32)21(35-23(31)13-18(25(33)34)12-22(29)30)11-16(2)9-7-5-6-8-10-19(27)14-20(28)15-26/h16-21,24,27-28,32H,4-15,26H2,1-3H3,(H,29,30)(H,33,34)/t16-,17+,18?,19+,20-,21-,24+/m0/s1
#> 10 InChI=1S/C25H47NO9/c1-4-17(3)24(32)21(35-23(31)13-18(25(33)34)12-22(29)30)11-16(2)9-7-5-6-8-10-19(27)14-20(28)15-26/h16-21,24,27-28,32H,4-15,26H2,1-3H3,(H,29,30)(H,33,34)/t16-,17+,18?,19+,20-,21-,24+/m0/s1
#> 11 InChI=1S/C25H47NO9/c1-4-17(3)24(32)21(35-23(31)13-18(25(33)34)12-22(29)30)11-16(2)9-7-5-6-8-10-19(27)14-20(28)15-26/h16-21,24,27-28,32H,4-15,26H2,1-3H3,(H,29,30)(H,33,34)/t16-,17+,18?,19+,20-,21-,24+/m0/s1
#> 12 InChI=1S/C25H47NO9/c1-4-17(3)24(32)21(35-23(31)13-18(25(33)34)12-22(29)30)11-16(2)9-7-5-6-8-10-19(27)14-20(28)15-26/h16-21,24,27-28,32H,4-15,26H2,1-3H3,(H,29,30)(H,33,34)/t16-,17+,18?,19+,20-,21-,24+/m0/s1
#> 13 InChI=1S/C25H47NO9/c1-4-17(3)24(32)21(35-23(31)13-18(25(33)34)12-22(29)30)11-16(2)9-7-5-6-8-10-19(27)14-20(28)15-26/h16-21,24,27-28,32H,4-15,26H2,1-3H3,(H,29,30)(H,33,34)/t16-,17+,18?,19+,20-,21-,24+/m0/s1
#> 14 InChI=1S/C25H47NO9/c1-4-17(3)24(32)21(35-23(31)13-18(25(33)34)12-22(29)30)11-16(2)9-7-5-6-8-10-19(27)14-20(28)15-26/h16-21,24,27-28,32H,4-15,26H2,1-3H3,(H,29,30)(H,33,34)/t16-,17+,18?,19+,20-,21-,24+/m0/s1
#> 15 InChI=1S/C25H47NO9/c1-4-17(3)24(32)21(35-23(31)13-18(25(33)34)12-22(29)30)11-16(2)9-7-5-6-8-10-19(27)14-20(28)15-26/h16-21,24,27-28,32H,4-15,26H2,1-3H3,(H,29,30)(H,33,34)/t16-,17+,18?,19+,20-,21-,24+/m0/s1
#> 16 InChI=1S/C17H12O6/c1-20-10-6-11-14(8-4-5-21-17(8)22-11)15-13(10)7-2-3-9(18)12(7)16(19)23-15/h4-6,8,17H,2-3H2,1H3/t8-,17+/m0/s1
#> 17 InChI=1S/C17H12O6/c1-20-10-6-11-14(8-4-5-21-17(8)22-11)15-13(10)7-2-3-9(18)12(7)16(19)23-15/h4-6,8,17H,2-3H2,1H3/t8-,17+/m0/s1
#> 18 InChI=1S/C17H12O6/c1-20-10-6-11-14(8-4-5-21-17(8)22-11)15-13(10)7-2-3-9(18)12(7)16(19)23-15/h4-6,8,17H,2-3H2,1H3/t8-,17+/m0/s1
#> 19 InChI=1S/C17H12O6/c1-20-10-6-11-14(8-4-5-21-17(8)22-11)15-13(10)7-2-3-9(18)12(7)16(19)23-15/h4-6,8,17H,2-3H2,1H3/t8-,17+/m0/s1
#> 20 InChI=1S/C17H12O6/c1-20-10-6-11-14(8-4-5-21-17(8)22-11)15-13(10)7-2-3-9(18)12(7)16(19)23-15/h4-6,8,17H,2-3H2,1H3/t8-,17+/m0/s1
#> 21 InChI=1S/C17H12O6/c1-20-10-6-11-14(8-4-5-21-17(8)22-11)15-13(10)7-2-3-9(18)12(7)16(19)23-15/h4-6,8,17H,2-3H2,1H3/t8-,17+/m0/s1
#> 22 InChI=1S/C17H12O6/c1-20-10-6-11-14(8-4-5-21-17(8)22-11)15-13(10)7-2-3-9(18)12(7)16(19)23-15/h4-6,8,17H,2-3H2,1H3/t8-,17+/m0/s1
#> 23 InChI=1S/C17H12O6/c1-20-10-6-11-14(8-4-5-21-17(8)22-11)15-13(10)7-2-3-9(18)12(7)16(19)23-15/h4-6,8,17H,2-3H2,1H3/t8-,17+/m0/s1
#> 24 InChI=1S/C17H12O6/c1-20-10-6-11-14(8-4-5-21-17(8)22-11)15-13(10)7-2-3-9(18)12(7)16(19)23-15/h4-6,8,17H,2-3H2,1H3/t8-,17+/m0/s1
#> 25 InChI=1S/C17H12O6/c1-20-10-6-11-14(8-4-5-21-17(8)22-11)15-13(10)7-2-3-9(18)12(7)16(19)23-15/h4-6,8,17H,2-3H2,1H3/t8-,17+/m0/s1
#> 26 InChI=1S/C17H14O6/c1-20-10-6-11-14(8-4-5-21-17(8)22-11)15-13(10)7-2-3-9(18)12(7)16(19)23-15/h6,8,17H,2-5H2,1H3/t8-,17+/m0/s1
#> 27 InChI=1S/C17H14O6/c1-20-10-6-11-14(8-4-5-21-17(8)22-11)15-13(10)7-2-3-9(18)12(7)16(19)23-15/h6,8,17H,2-5H2,1H3/t8-,17+/m0/s1
#> 28 InChI=1S/C17H14O6/c1-20-10-6-11-14(8-4-5-21-17(8)22-11)15-13(10)7-2-3-9(18)12(7)16(19)23-15/h6,8,17H,2-5H2,1H3/t8-,17+/m0/s1
#> 29 InChI=1S/C17H14O6/c1-20-10-6-11-14(8-4-5-21-17(8)22-11)15-13(10)7-2-3-9(18)12(7)16(19)23-15/h6,8,17H,2-5H2,1H3/t8-,17+/m0/s1
#> 30 InChI=1S/C17H14O6/c1-20-10-6-11-14(8-4-5-21-17(8)22-11)15-13(10)7-2-3-9(18)12(7)16(19)23-15/h6,8,17H,2-5H2,1H3/t8-,17+/m0/s1
#> 31 InChI=1S/C17H14O6/c1-20-10-6-11-14(8-4-5-21-17(8)22-11)15-13(10)7-2-3-9(18)12(7)16(19)23-15/h6,8,17H,2-5H2,1H3/t8-,17+/m0/s1
#> 32 InChI=1S/C17H12O7/c1-20-9-6-10-12(8-3-5-22-17(8)23-10)14-11(9)7-2-4-21-15(18)13(7)16(19)24-14/h3,5-6,8,17H,2,4H2,1H3/t8-,17+/m0/s1
#> 33 InChI=1S/C17H12O7/c1-20-9-6-10-12(8-3-5-22-17(8)23-10)14-11(9)7-2-4-21-15(18)13(7)16(19)24-14/h3,5-6,8,17H,2,4H2,1H3/t8-,17+/m0/s1
#> 34 InChI=1S/C17H12O7/c1-20-9-6-10-12(8-3-5-22-17(8)23-10)14-11(9)7-2-4-21-15(18)13(7)16(19)24-14/h3,5-6,8,17H,2,4H2,1H3/t8-,17+/m0/s1
#> 35 InChI=1S/C17H12O7/c1-20-9-6-10-12(8-3-5-22-17(8)23-10)14-11(9)7-2-4-21-15(18)13(7)16(19)24-14/h3,5-6,8,17H,2,4H2,1H3/t8-,17+/m0/s1
#> 36 InChI=1S/C17H12O7/c1-20-9-6-10-12(8-3-5-22-17(8)23-10)14-11(9)7-2-4-21-15(18)13(7)16(19)24-14/h3,5-6,8,17H,2,4H2,1H3/t8-,17+/m0/s1
#> 37 InChI=1S/C17H12O7/c1-20-9-6-10-12(8-3-5-22-17(8)23-10)14-11(9)7-2-4-21-15(18)13(7)16(19)24-14/h3,5-6,8,17H,2,4H2,1H3/t8-,17+/m0/s1
#> 38 InChI=1S/C17H14O7/c1-20-9-6-10-12(8-3-5-22-17(8)23-10)14-11(9)7-2-4-21-15(18)13(7)16(19)24-14/h6,8,17H,2-5H2,1H3/t8-,17+/m0/s1
#> 39 InChI=1S/C17H14O7/c1-20-9-6-10-12(8-3-5-22-17(8)23-10)14-11(9)7-2-4-21-15(18)13(7)16(19)24-14/h6,8,17H,2-5H2,1H3/t8-,17+/m0/s1
#> 40 InChI=1S/C17H14O7/c1-20-9-6-10-12(8-3-5-22-17(8)23-10)14-11(9)7-2-4-21-15(18)13(7)16(19)24-14/h6,8,17H,2-5H2,1H3/t8-,17+/m0/s1
#> 41 InChI=1S/C17H14O7/c1-20-9-6-10-12(8-3-5-22-17(8)23-10)14-11(9)7-2-4-21-15(18)13(7)16(19)24-14/h6,8,17H,2-5H2,1H3/t8-,17+/m0/s1
#> 42 InChI=1S/C17H14O7/c1-20-9-6-10-12(8-3-5-22-17(8)23-10)14-11(9)7-2-4-21-15(18)13(7)16(19)24-14/h6,8,17H,2-5H2,1H3/t8-,17+/m0/s1
#> 43 InChI=1S/C17H14O7/c1-20-9-6-10-12(8-3-5-22-17(8)23-10)14-11(9)7-2-4-21-15(18)13(7)16(19)24-14/h6,8,17H,2-5H2,1H3/t8-,17+/m0/s1
#> 44 InChI=1S/C17H12O7/c1-21-9-6-10-13(17(20)4-5-22-16(17)23-10)14-12(9)7-2-3-8(18)11(7)15(19)24-14/h4-6,16,20H,2-3H2,1H3/t16-,17-/m1/s1
#> 45 InChI=1S/C17H12O7/c1-21-9-6-10-13(17(20)4-5-22-16(17)23-10)14-12(9)7-2-3-8(18)11(7)15(19)24-14/h4-6,16,20H,2-3H2,1H3/t16-,17-/m1/s1
#> 46 InChI=1S/C17H12O7/c1-21-9-6-10-13(17(20)4-5-22-16(17)23-10)14-12(9)7-2-3-8(18)11(7)15(19)24-14/h4-6,16,20H,2-3H2,1H3/t16-,17-/m1/s1
#> 47 InChI=1S/C17H12O7/c1-21-9-6-10-13(17(20)4-5-22-16(17)23-10)14-12(9)7-2-3-8(18)11(7)15(19)24-14/h4-6,16,20H,2-3H2,1H3/t16-,17-/m1/s1
#> 48 InChI=1S/C17H12O7/c1-21-9-6-10-13(17(20)4-5-22-16(17)23-10)14-12(9)7-2-3-8(18)11(7)15(19)24-14/h4-6,16,20H,2-3H2,1H3/t16-,17-/m1/s1
#> 49 InChI=1S/C17H12O7/c1-21-9-6-10-13(17(20)4-5-22-16(17)23-10)14-12(9)7-2-3-8(18)11(7)15(19)24-14/h4-6,16,20H,2-3H2,1H3/t16-,17-/m1/s1
#> 50 InChI=1S/C20H20N2O3/c1-9(23)14-18(24)17-16-11-8-21-13-6-4-5-10(15(11)13)7-12(16)20(2,3)22(17)19(14)25/h4-6,8,12,16-17,21,24H,7H2,1-3H3/t12-,16+,17+/m1/s1
#> 51 InChI=1S/C20H20N2O3/c1-9(23)14-18(24)17-16-11-8-21-13-6-4-5-10(15(11)13)7-12(16)20(2,3)22(17)19(14)25/h4-6,8,12,16-17,21,24H,7H2,1-3H3/t12-,16+,17+/m1/s1
#> 52 InChI=1S/C20H20N2O3/c1-9(23)14-18(24)17-16-11-8-21-13-6-4-5-10(15(11)13)7-12(16)20(2,3)22(17)19(14)25/h4-6,8,12,16-17,21,24H,7H2,1-3H3/t12-,16+,17+/m1/s1
#> 53 InChI=1S/C20H20N2O3/c1-9(23)14-18(24)17-16-11-8-21-13-6-4-5-10(15(11)13)7-12(16)20(2,3)22(17)19(14)25/h4-6,8,12,16-17,21,24H,7H2,1-3H3/t12-,16+,17+/m1/s1
#> 54 InChI=1S/C20H20N2O3/c1-9(23)14-18(24)17-16-11-8-21-13-6-4-5-10(15(11)13)7-12(16)20(2,3)22(17)19(14)25/h4-6,8,12,16-17,21,24H,7H2,1-3H3/t12-,16+,17+/m1/s1
#> 55 InChI=1S/C15H16O6/c1-15-6-12(18)10(16)5-9(15)8-3-7(20-2)4-11(17)13(8)14(19)21-15/h3-5,10,12,16-18H,6H2,1-2H3/t10-,12-,15-/m0/s1
#> 56 InChI=1S/C15H16O6/c1-15-6-12(18)10(16)5-9(15)8-3-7(20-2)4-11(17)13(8)14(19)21-15/h3-5,10,12,16-18H,6H2,1-2H3/t10-,12-,15-/m0/s1
#> 57 InChI=1S/C15H16O6/c1-15-6-12(18)10(16)5-9(15)8-3-7(20-2)4-11(17)13(8)14(19)21-15/h3-5,10,12,16-18H,6H2,1-2H3/t10-,12-,15-/m0/s1
#> 58 InChI=1S/C15H16O6/c1-15-6-12(18)10(16)5-9(15)8-3-7(20-2)4-11(17)13(8)14(19)21-15/h3-5,10,12,16-18H,6H2,1-2H3/t10-,12-,15-/m0/s1
#> 59 InChI=1S/C15H16O6/c1-15-6-12(18)10(16)5-9(15)8-3-7(20-2)4-11(17)13(8)14(19)21-15/h3-5,10,12,16-18H,6H2,1-2H3/t10-,12-,15-/m0/s1
#> 60 InChI=1S/C14H10O5/c1-6-2-7(15)5-11-12(6)9-3-8(16)4-10(17)13(9)14(18)19-11/h2-5,15-17H,1H3
#> 61 InChI=1S/C14H10O5/c1-6-2-7(15)5-11-12(6)9-3-8(16)4-10(17)13(9)14(18)19-11/h2-5,15-17H,1H3
#> 62 InChI=1S/C14H10O5/c1-6-2-7(15)5-11-12(6)9-3-8(16)4-10(17)13(9)14(18)19-11/h2-5,15-17H,1H3
#> 63 InChI=1S/C15H12O5/c1-7-3-8(16)4-12-13(7)10-5-9(19-2)6-11(17)14(10)15(18)20-12/h3-6,16-17H,1-2H3
#> 64 InChI=1S/C15H12O5/c1-7-3-8(16)4-12-13(7)10-5-9(19-2)6-11(17)14(10)15(18)20-12/h3-6,16-17H,1-2H3
#> 65 InChI=1S/C15H12O5/c1-7-3-8(16)4-12-13(7)10-5-9(19-2)6-11(17)14(10)15(18)20-12/h3-6,16-17H,1-2H3
#> 66 InChI=1S/C16H16O8/c1-16(23)14(21)10-9(13(20)15(16)22)12(19)8-6(11(10)18)3-5(24-2)4-7(8)17/h3-4,13-15,17,20-23H,1-2H3/t13-,14+,15+,16-/m0/s1
#> 67 InChI=1S/C16H16O8/c1-16(23)14(21)10-9(13(20)15(16)22)12(19)8-6(11(10)18)3-5(24-2)4-7(8)17/h3-4,13-15,17,20-23H,1-2H3/t13-,14+,15+,16-/m0/s1
#> 68 InChI=1S/C16H16O8/c1-16(23)14(21)10-9(13(20)15(16)22)12(19)8-6(11(10)18)3-5(24-2)4-7(8)17/h3-4,13-15,17,20-23H,1-2H3/t13-,14+,15+,16-/m0/s1
#> 69 InChI=1S/C16H16O8/c1-16(23)14(21)10-9(13(20)15(16)22)12(19)8-6(11(10)18)3-5(24-2)4-7(8)17/h3-4,13-15,17,20-23H,1-2H3/t13-,14+,15+,16-/m0/s1
#> 70 InChI=1S/C16H16O8/c1-16(23)14(21)10-9(13(20)15(16)22)12(19)8-6(11(10)18)3-5(24-2)4-7(8)17/h3-4,13-15,17,20-23H,1-2H3/t13-,14+,15+,16-/m0/s1
#> inchikey cas pubchem
#> 1 KWILGNNWGSNMPA-UHFFFAOYSA-N 17397-85-2 CID:28516
#> 2 KWILGNNWGSNMPA-UHFFFAOYSA-N 17397-85-2 CID:28516
#> 3 KWILGNNWGSNMPA-UHFFFAOYSA-N 17397-85-2 CID:28516
#> 4 KWILGNNWGSNMPA-UHFFFAOYSA-N 17397-85-2 CID:28516
#> 5 KWILGNNWGSNMPA-UHFFFAOYSA-N 17397-85-2 CID:28516
#> 6 CTXQVLLVFBNZKL-YVEDVMJTSA-N 149849-90-1 CID:102004382
#> 7 CTXQVLLVFBNZKL-YVEDVMJTSA-N 149849-90-1 CID:102004382
#> 8 CTXQVLLVFBNZKL-YVEDVMJTSA-N 149849-90-1 CID:102004382
#> 9 CTXQVLLVFBNZKL-YVEDVMJTSA-N 149849-90-1 CID:102004382
#> 10 CTXQVLLVFBNZKL-YVEDVMJTSA-N 149849-90-1 CID:102004382
#> 11 CTXQVLLVFBNZKL-YVEDVMJTSA-N 149849-90-1 CID:102004382
#> 12 CTXQVLLVFBNZKL-YVEDVMJTSA-N 149849-90-1 CID:102004382
#> 13 CTXQVLLVFBNZKL-YVEDVMJTSA-N 149849-90-1 CID:102004382
#> 14 CTXQVLLVFBNZKL-YVEDVMJTSA-N 149849-90-1 CID:102004382
#> 15 CTXQVLLVFBNZKL-YVEDVMJTSA-N 149849-90-1 CID:102004382
#> 16 OQIQSTLJSLGHID-WNWIJWBNSA-N 1162-65-8 CID:186907
#> 17 OQIQSTLJSLGHID-WNWIJWBNSA-N 1162-65-8 CID:186907
#> 18 OQIQSTLJSLGHID-WNWIJWBNSA-N 1162-65-8 CID:186907
#> 19 OQIQSTLJSLGHID-WNWIJWBNSA-N 1162-65-8 CID:186907
#> 20 OQIQSTLJSLGHID-WNWIJWBNSA-N 1162-65-8 CID:186907
#> 21 OQIQSTLJSLGHID-WNWIJWBNSA-N 1162-65-8 CID:186907
#> 22 OQIQSTLJSLGHID-WNWIJWBNSA-N 1162-65-8 CID:186907
#> 23 OQIQSTLJSLGHID-WNWIJWBNSA-N 1162-65-8 CID:186907
#> 24 OQIQSTLJSLGHID-WNWIJWBNSA-N 1162-65-8 CID:186907
#> 25 OQIQSTLJSLGHID-WNWIJWBNSA-N 1162-65-8 CID:186907
#> 26 WWSYXEZEXMQWHT-WNWIJWBNSA-N 7220-81-7 CID:2724360
#> 27 WWSYXEZEXMQWHT-WNWIJWBNSA-N 7220-81-7 CID:2724360
#> 28 WWSYXEZEXMQWHT-WNWIJWBNSA-N 7220-81-7 CID:2724360
#> 29 WWSYXEZEXMQWHT-WNWIJWBNSA-N 7220-81-7 CID:2724360
#> 30 WWSYXEZEXMQWHT-WNWIJWBNSA-N 7220-81-7 CID:2724360
#> 31 WWSYXEZEXMQWHT-WNWIJWBNSA-N 7220-81-7 CID:2724360
#> 32 XWIYFDMXXLINPU-WNWIJWBNSA-N 1165-39-5 CID:2724361
#> 33 XWIYFDMXXLINPU-WNWIJWBNSA-N 1165-39-5 CID:2724361
#> 34 XWIYFDMXXLINPU-WNWIJWBNSA-N 1165-39-5 CID:2724361
#> 35 XWIYFDMXXLINPU-WNWIJWBNSA-N 1165-39-5 CID:2724361
#> 36 XWIYFDMXXLINPU-WNWIJWBNSA-N 1165-39-5 CID:2724361
#> 37 XWIYFDMXXLINPU-WNWIJWBNSA-N 1165-39-5 CID:2724361
#> 38 WPCVRWVBBXIRMA-WNWIJWBNSA-N 7241-98-7 CID:2724362
#> 39 WPCVRWVBBXIRMA-WNWIJWBNSA-N 7241-98-7 CID:2724362
#> 40 WPCVRWVBBXIRMA-WNWIJWBNSA-N 7241-98-7 CID:2724362
#> 41 WPCVRWVBBXIRMA-WNWIJWBNSA-N 7241-98-7 CID:2724362
#> 42 WPCVRWVBBXIRMA-WNWIJWBNSA-N 7241-98-7 CID:2724362
#> 43 WPCVRWVBBXIRMA-WNWIJWBNSA-N 7241-98-7 CID:2724362
#> 44 MJBWDEQAUQTVKK-IAGOWNOFSA-N 6795-23-9 CID:15558498
#> 45 MJBWDEQAUQTVKK-IAGOWNOFSA-N 6795-23-9 CID:15558498
#> 46 MJBWDEQAUQTVKK-IAGOWNOFSA-N 6795-23-9 CID:15558498
#> 47 MJBWDEQAUQTVKK-IAGOWNOFSA-N 6795-23-9 CID:15558498
#> 48 MJBWDEQAUQTVKK-IAGOWNOFSA-N 6795-23-9 CID:15558498
#> 49 MJBWDEQAUQTVKK-IAGOWNOFSA-N 6795-23-9 CID:15558498
#> 50 SZINUGQCTHLQAZ-DQYPLSBCSA-N 18172-33-3 CID:54682463
#> 51 SZINUGQCTHLQAZ-DQYPLSBCSA-N 18172-33-3 CID:54682463
#> 52 SZINUGQCTHLQAZ-DQYPLSBCSA-N 18172-33-3 CID:54682463
#> 53 SZINUGQCTHLQAZ-DQYPLSBCSA-N 18172-33-3 CID:54682463
#> 54 SZINUGQCTHLQAZ-DQYPLSBCSA-N 18172-33-3 CID:54682463
#> 55 MMHTXEATDNFMMY-WBIUFABUSA-N 29752-43-0 CID:34687
#> 56 MMHTXEATDNFMMY-WBIUFABUSA-N 29752-43-0 CID:34687
#> 57 MMHTXEATDNFMMY-WBIUFABUSA-N 29752-43-0 CID:34687
#> 58 MMHTXEATDNFMMY-WBIUFABUSA-N 29752-43-0 CID:34687
#> 59 MMHTXEATDNFMMY-WBIUFABUSA-N 29752-43-0 CID:34687
#> 60 CEBXXEKPIIDJHL-UHFFFAOYSA-N 641-38-3 CID:5359485
#> 61 CEBXXEKPIIDJHL-UHFFFAOYSA-N 641-38-3 CID:5359485
#> 62 CEBXXEKPIIDJHL-UHFFFAOYSA-N 641-38-3 CID:5359485
#> 63 LCSDQFNUYFTXMT-UHFFFAOYSA-N 23452-05-3 CID:5360741
#> 64 LCSDQFNUYFTXMT-UHFFFAOYSA-N 23452-05-3 CID:5360741
#> 65 LCSDQFNUYFTXMT-UHFFFAOYSA-N 23452-05-3 CID:5360741
#> 66 VSMBLBOUQJNJIL-JJXSEGSLSA-N 22268-16-2 CID:89644
#> 67 VSMBLBOUQJNJIL-JJXSEGSLSA-N 22268-16-2 CID:89644
#> 68 VSMBLBOUQJNJIL-JJXSEGSLSA-N 22268-16-2 CID:89644
#> 69 VSMBLBOUQJNJIL-JJXSEGSLSA-N 22268-16-2 CID:89644
#> 70 VSMBLBOUQJNJIL-JJXSEGSLSA-N 22268-16-2 CID:89644
#> name
#> 1 Mellein
#> 2 Mellein
#> 3 Mellein
#> 4 Mellein
#> 5 Mellein
#> 6 AAL toxin TB
#> 7 AAL toxin TB
#> 8 AAL toxin TB
#> 9 AAL toxin TB
#> 10 AAL toxin TB
#> 11 AAL toxin TB
#> 12 AAL toxin TB
#> 13 AAL toxin TB
#> 14 AAL toxin TB
#> 15 AAL toxin TB
#> 16 Aflatoxin B1
#> 17 Aflatoxin B1
#> 18 Aflatoxin B1
#> 19 Aflatoxin B1
#> 20 Aflatoxin B1
#> 21 Aflatoxin B1
#> 22 Aflatoxin B1
#> 23 Aflatoxin B1
#> 24 Aflatoxin B1
#> 25 Aflatoxin B1
#> 26 Aflatoxin B2
#> 27 Aflatoxin B2
#> 28 Aflatoxin B2
#> 29 Aflatoxin B2
#> 30 Aflatoxin B2
#> 31 Aflatoxin B2
#> 32 Aflatoxin G1
#> 33 Aflatoxin G1
#> 34 Aflatoxin G1
#> 35 Aflatoxin G1
#> 36 Aflatoxin G1
#> 37 Aflatoxin G1
#> 38 Aflatoxin G2
#> 39 Aflatoxin G2
#> 40 Aflatoxin G2
#> 41 Aflatoxin G2
#> 42 Aflatoxin G2
#> 43 Aflatoxin G2
#> 44 Aflatoxin M1
#> 45 Aflatoxin M1
#> 46 Aflatoxin M1
#> 47 Aflatoxin M1
#> 48 Aflatoxin M1
#> 49 Aflatoxin M1
#> 50 alpha-Cyclopiazonic acid
#> 51 alpha-Cyclopiazonic acid
#> 52 alpha-Cyclopiazonic acid
#> 53 alpha-Cyclopiazonic acid
#> 54 alpha-Cyclopiazonic acid
#> 55 Altenuene
#> 56 Altenuene
#> 57 Altenuene
#> 58 Altenuene
#> 59 Altenuene
#> 60 Alternariol
#> 61 Alternariol
#> 62 Alternariol
#> 63 Alternariol methyl ether
#> 64 Alternariol methyl ether
#> 65 Alternariol methyl ether
#> 66 Altersolanol A
#> 67 Altersolanol A
#> 68 Altersolanol A
#> 69 Altersolanol A
#> 70 Altersolanol A
## Add also the synonyms (aliases) for the compounds. This will cause the
## tables compound and synonym to be joined. The elements of the compound_id
## and name are now no longer unique
res <- compounds(cdb, columns = c("name", "synonym"))
head(res)
#> name
#> 1 Mellein
#> 2 Mellein
#> 3 Mellein
#> 4 AAL toxin TB
#> 5 AAL toxin TB
#> 6 Aflatoxin B1
#> synonym
#> 1 8-hydroxy-3-methyl-3,4-dihydroisochromen-1-one
#> 2 Mellein
#> 3 Ochracin
#> 4 2-[2-[(3R,4R,5S,7S,14R,16S)-17-amino-4,14,16-trihydroxy-3,7-dimethylheptadecan-5-yl]oxy-2-oxoethyl]butanedioic acid
#> 5 AAL toxin TB
#> 6 (6aR,9aS)-4-Methoxy-2,3,6a,9a-tetrahydrocyclopenta[c]furo[3',2':4,5]furo[2,3-h]chromene-1,11-dione
## List all database tables and their columns
tables(cdb)
#> $ms_compound
#> [1] "compound_id" "formula" "exactmass" "smiles" "inchi"
#> [6] "inchikey" "cas" "pubchem" "name"
#>
#> $msms_spectrum
#> [1] "accession" "spectrum_name" "date"
#> [4] "authors" "license" "copyright"
#> [7] "publication" "ms_level" "polarity"
#> [10] "splash" "compound_id" "precursor_intensity"
#> [13] "precursor_mz" "adduct" "ionization"
#> [16] "ionization_voltage" "fragmentation_mode" "collision_energy_text"
#> [19] "instrument" "instrument_type" "precursor_mz_text"
#> [22] "spectrum_id" "collision_energy" "predicted"
#> [25] "msms_mz_range_min" "msms_mz_range_max"
#>
#> $msms_spectrum_peak
#> [1] "spectrum_id" "mz" "intensity" "peak_id"
#>
#> $synonym
#> [1] "compound_id" "synonym"
#>
## Any of these columns can be used in the `compounds` call to retrieve
## the specific annotations. The corresponding database tables will then be
## joined together
compounds(cdb, columns = c("formula", "publication"))
#> formula
#> 1 C10H10O3
#> 2 C25H47NO9
#> 3 C17H12O6
#> 4 C17H14O6
#> 5 C17H12O7
#> 6 C17H14O7
#> 7 C20H20N2O3
#> 8 C15H16O6
#> 9 C14H10O5
#> 10 C15H12O5
#> 11 C16H16O8
#> publication
#> 1 Renaud, J. B.; Sumarah, M. W. Data Independent Acquisition-Digital Archiving Mass Spectrometry: Application to Single Kernel Mycotoxin Analysis of Fusarium Graminearum Infected Maize. Analytical and Bioanalytical Chemistry 2016, 408 (12), 3083–91. DOI:10.1007/s00216-016-9391-5
#> 2 Renaud, J. B.; Kelman, M. J.; Qi, T. F.; Seifert, K. A.; Sumarah, M. W. Product Ion Filtering with Rapid Polarity Switching for the Detection of All Fumonisins and AAL-Toxins. Rapid Communications in Mass Spectrometry 2015, 29 (22), 2131–9. DOI:10.1002/rcm.7374
#> 3 Renaud, J. B.; Sumarah, M. W. Data Independent Acquisition-Digital Archiving Mass Spectrometry: Application to Single Kernel Mycotoxin Analysis of Fusarium Graminearum Infected Maize. Analytical and Bioanalytical Chemistry 2016, 408 (12), 3083–91. DOI:10.1007/s00216-016-9391-5
#> 4 Renaud, J. B.; Sumarah, M. W. Data Independent Acquisition-Digital Archiving Mass Spectrometry: Application to Single Kernel Mycotoxin Analysis of Fusarium Graminearum Infected Maize. Analytical and Bioanalytical Chemistry 2016, 408 (12), 3083–91. DOI:10.1007/s00216-016-9391-5
#> 5 Renaud, J. B.; Sumarah, M. W. Data Independent Acquisition-Digital Archiving Mass Spectrometry: Application to Single Kernel Mycotoxin Analysis of Fusarium Graminearum Infected Maize. Analytical and Bioanalytical Chemistry 2016, 408 (12), 3083–91. DOI:10.1007/s00216-016-9391-5
#> 6 Renaud, J. B.; Sumarah, M. W. Data Independent Acquisition-Digital Archiving Mass Spectrometry: Application to Single Kernel Mycotoxin Analysis of Fusarium Graminearum Infected Maize. Analytical and Bioanalytical Chemistry 2016, 408 (12), 3083–91. DOI:10.1007/s00216-016-9391-5
#> 7 Renaud, J. B.; Sumarah, M. W. Data Independent Acquisition-Digital Archiving Mass Spectrometry: Application to Single Kernel Mycotoxin Analysis of Fusarium Graminearum Infected Maize. Analytical and Bioanalytical Chemistry 2016, 408 (12), 3083–91. DOI:10.1007/s00216-016-9391-5
#> 8 <NA>
#> 9 Renaud, J. B.; Sumarah, M. W. Data Independent Acquisition-Digital Archiving Mass Spectrometry: Application to Single Kernel Mycotoxin Analysis of Fusarium Graminearum Infected Maize. Analytical and Bioanalytical Chemistry 2016, 408 (12), 3083–91. DOI:10.1007/s00216-016-9391-5
#> 10 Renaud, J. B.; Sumarah, M. W. Data Independent Acquisition-Digital Archiving Mass Spectrometry: Application to Single Kernel Mycotoxin Analysis of Fusarium Graminearum Infected Maize. Analytical and Bioanalytical Chemistry 2016, 408 (12), 3083–91. DOI:10.1007/s00216-016-9391-5
#> 11 Renaud, J. B.; Sumarah, M. W. Data Independent Acquisition-Digital Archiving Mass Spectrometry: Application to Single Kernel Mycotoxin Analysis of Fusarium Graminearum Infected Maize. Analytical and Bioanalytical Chemistry 2016, 408 (12), 3083–91. DOI:10.1007/s00216-016-9391-5
## Calculating m/z values for the exact masses of unique chemical formulas
## in the database:
mass2mz(cdb, adduct = c("[M+H]+", "[M+Na]+"))
#> [M+H]+ [M+Na]+
#> C10H10O3 179.0703 201.0522
#> C25H47NO9 506.3324 528.3143
#> C17H12O6 313.0706 335.0526
#> C17H14O6 315.0863 337.0682
#> C17H12O7 329.0656 351.0475
#> C17H14O7 331.0812 353.0632
#> C20H20N2O3 337.1547 359.1366
#> C15H16O6 293.1020 315.0839
#> C14H10O5 259.0601 281.0420
#> C15H12O5 273.0757 295.0577
#> C16H16O8 337.0918 359.0737
## By using `name = "compound_id"` the calculation will be performed for
## each unique compound ID instead (resulting in potentially redundant
## results)
mass2mz(cdb, adduct = c("[M+H]+", "[M+Na]+"), name = "compound_id")
#> [M+H]+ [M+Na]+
#> 1 179.0703 201.0522
#> 2 179.0703 201.0522
#> 3 179.0703 201.0522
#> 4 179.0703 201.0522
#> 5 179.0703 201.0522
#> 6 506.3324 528.3143
#> 7 506.3324 528.3143
#> 8 506.3324 528.3143
#> 9 506.3324 528.3143
#> 10 506.3324 528.3143
#> 11 506.3324 528.3143
#> 12 506.3324 528.3143
#> 13 506.3324 528.3143
#> 14 506.3324 528.3143
#> 15 506.3324 528.3143
#> 16 313.0706 335.0526
#> 17 313.0706 335.0526
#> 18 313.0706 335.0526
#> 19 313.0706 335.0526
#> 20 313.0706 335.0526
#> 21 313.0706 335.0526
#> 22 313.0706 335.0526
#> 23 313.0706 335.0526
#> 24 313.0706 335.0526
#> 25 313.0706 335.0526
#> 26 315.0863 337.0682
#> 27 315.0863 337.0682
#> 28 315.0863 337.0682
#> 29 315.0863 337.0682
#> 30 315.0863 337.0682
#> 31 315.0863 337.0682
#> 32 329.0656 351.0475
#> 33 329.0656 351.0475
#> 34 329.0656 351.0475
#> 35 329.0656 351.0475
#> 36 329.0656 351.0475
#> 37 329.0656 351.0475
#> 38 331.0812 353.0632
#> 39 331.0812 353.0632
#> 40 331.0812 353.0632
#> 41 331.0812 353.0632
#> 42 331.0812 353.0632
#> 43 331.0812 353.0632
#> 44 329.0656 351.0475
#> 45 329.0656 351.0475
#> 46 329.0656 351.0475
#> 47 329.0656 351.0475
#> 48 329.0656 351.0475
#> 49 329.0656 351.0475
#> 50 337.1547 359.1366
#> 51 337.1547 359.1366
#> 52 337.1547 359.1366
#> 53 337.1547 359.1366
#> 54 337.1547 359.1366
#> 55 293.1020 315.0839
#> 56 293.1020 315.0839
#> 57 293.1020 315.0839
#> 58 293.1020 315.0839
#> 59 293.1020 315.0839
#> 60 259.0601 281.0420
#> 61 259.0601 281.0420
#> 62 259.0601 281.0420
#> 63 273.0757 295.0577
#> 64 273.0757 295.0577
#> 65 273.0757 295.0577
#> 66 337.0918 359.0737
#> 67 337.0918 359.0737
#> 68 337.0918 359.0737
#> 69 337.0918 359.0737
#> 70 337.0918 359.0737
## Create a Spectra object with all MS/MS spectra from the database.
library(Spectra)
#> Loading required package: BiocParallel
sps <- Spectra(cdb)
sps
#> MSn data (Spectra) with 70 spectra in a MsBackendCompDb backend:
#> msLevel precursorMz polarity
#> <integer> <numeric> <integer>
#> 1 2 179.07 1
#> 2 2 179.07 1
#> 3 2 179.07 1
#> 4 2 179.07 1
#> 5 2 179.07 1
#> ... ... ... ...
#> 66 2 337.091 1
#> 67 2 337.091 1
#> 68 2 337.091 1
#> 69 2 337.091 1
#> 70 2 337.091 1
#> ... 47 more variables/columns.
#> Use 'spectraVariables' to list all of them.
#> data source: MassBank
#> version: 2020.09
#> organism: NA
## Extract spectra for a specific compound.
sps <- Spectra(cdb, filter = ~ name == "Mellein")
sps
#> MSn data (Spectra) with 5 spectra in a MsBackendCompDb backend:
#> msLevel precursorMz polarity
#> <integer> <numeric> <integer>
#> 1 2 179.07 1
#> 2 2 179.07 1
#> 3 2 179.07 1
#> 4 2 179.07 1
#> 5 2 179.07 1
#> ... 47 more variables/columns.
#> Use 'spectraVariables' to list all of them.
#> data source: MassBank
#> version: 2020.09
#> organism: NA
## List all available annotations for MS/MS spectra
spectraVariables(sps)
#> [1] "msLevel" "rtime"
#> [3] "acquisitionNum" "scanIndex"
#> [5] "dataStorage" "dataOrigin"
#> [7] "centroided" "smoothed"
#> [9] "polarity" "precScanNum"
#> [11] "precursorMz" "precursorIntensity"
#> [13] "precursorCharge" "collisionEnergy"
#> [15] "isolationWindowLowerMz" "isolationWindowTargetMz"
#> [17] "isolationWindowUpperMz" "compound_id"
#> [19] "name" "formula"
#> [21] "exactmass" "smiles"
#> [23] "inchi" "inchikey"
#> [25] "cas" "pubchem"
#> [27] "accession" "spectrum_name"
#> [29] "date" "authors"
#> [31] "license" "copyright"
#> [33] "publication" "splash"
#> [35] "precursor_intensity" "adduct"
#> [37] "ionization" "ionization_voltage"
#> [39] "fragmentation_mode" "collisionEnergy_text"
#> [41] "instrument" "instrument_type"
#> [43] "precursorMz_text" "spectrum_id"
#> [45] "predicted" "msms_mz_range_min"
#> [47] "msms_mz_range_max" "synonym"
## Get access to the m/z values of these
mz(sps)
#> NumericList of length 5
#> [[1]] 133.0648 151.0754 155.9743 161.0597 179.0703
#> [[2]] 133.0648 151.0754 155.9745 161.0597 179.0703
#> [[3]] 105.0699 133.0648 151.0754 161.0597 179.0703
#> [[4]] 105.0699 133.0648 151.0754 161.0597 179.0703
#> [[5]] 105.0699 115.0542 133.0648 151.0754 161.0597 179.0703
library(Spectra)
## Plot the first spectrum
plotSpectra(sps[1])
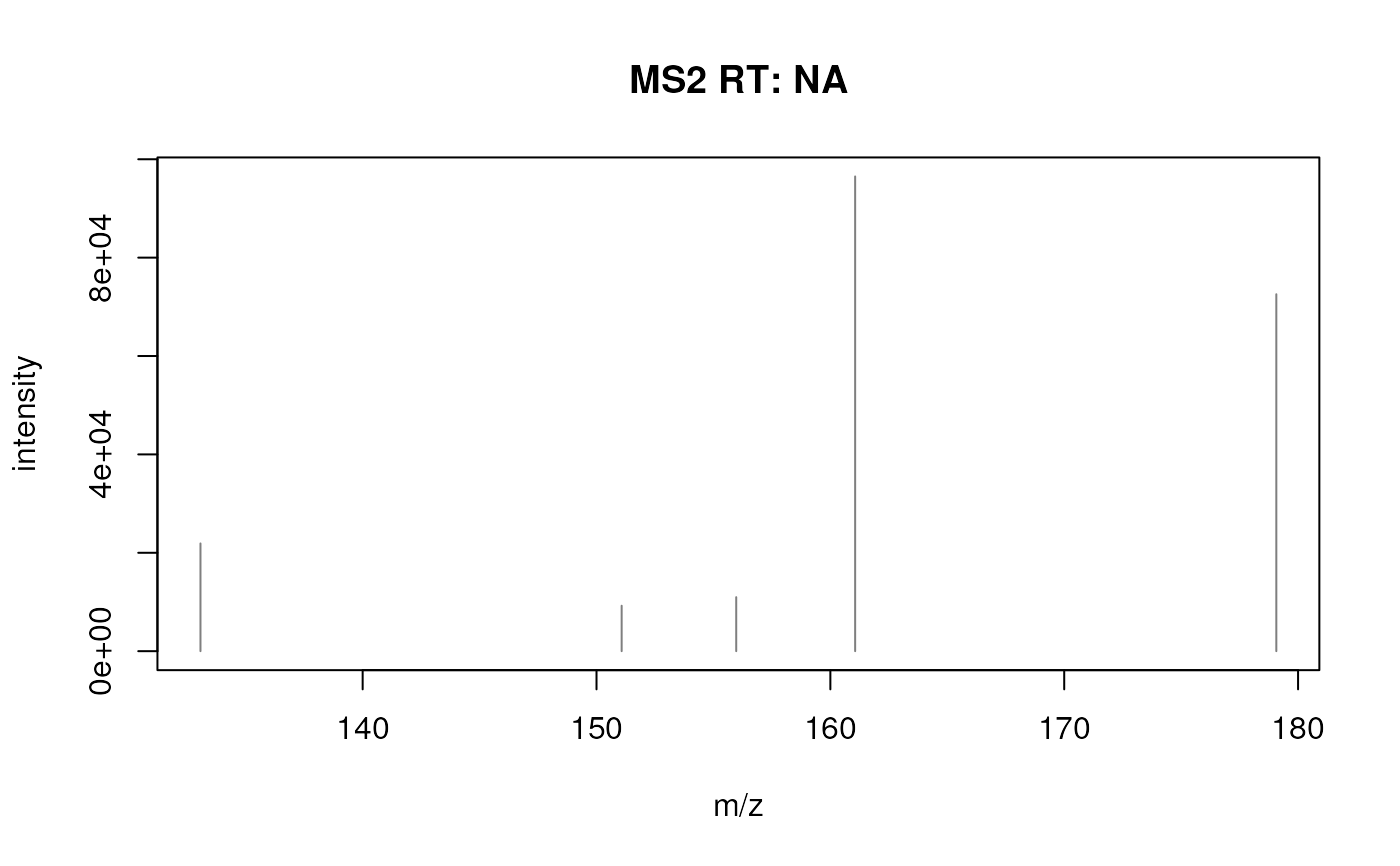 #########
## Filtering the database
##
## Get all compounds with an exact mass between 310 and 320
res <- compounds(cdb, filter = ~ exactmass > 310 & exactmass < 320)
res
#> formula exactmass smiles
#> 1 C17H12O6 312.0634 COC1=C2C3=C(C(=O)CC3)C(=O)OC2=C4[C@@H]5C=CO[C@@H]5OC4=C1
#> 2 C17H14O6 314.0790 COC1=C2C3=C(C(=O)CC3)C(=O)OC2=C4[C@@H]5CCO[C@@H]5OC4=C1
#> inchi
#> 1 InChI=1S/C17H12O6/c1-20-10-6-11-14(8-4-5-21-17(8)22-11)15-13(10)7-2-3-9(18)12(7)16(19)23-15/h4-6,8,17H,2-3H2,1H3/t8-,17+/m0/s1
#> 2 InChI=1S/C17H14O6/c1-20-10-6-11-14(8-4-5-21-17(8)22-11)15-13(10)7-2-3-9(18)12(7)16(19)23-15/h6,8,17H,2-5H2,1H3/t8-,17+/m0/s1
#> inchikey cas pubchem name
#> 1 OQIQSTLJSLGHID-WNWIJWBNSA-N 1162-65-8 CID:186907 Aflatoxin B1
#> 2 WWSYXEZEXMQWHT-WNWIJWBNSA-N 7220-81-7 CID:2724360 Aflatoxin B2
## Get all compounds that have an H14 in their formula.
res <- compounds(cdb, filter = FormulaFilter("H14", "contains"))
res
#> formula exactmass smiles
#> 1 C17H14O6 314.0790 COC1=C2C3=C(C(=O)CC3)C(=O)OC2=C4[C@@H]5CCO[C@@H]5OC4=C1
#> 2 C17H14O7 330.0739 COC1=C2C3=C(C(=O)OCC3)C(=O)OC2=C4[C@@H]5CCO[C@@H]5OC4=C1
#> inchi
#> 1 InChI=1S/C17H14O6/c1-20-10-6-11-14(8-4-5-21-17(8)22-11)15-13(10)7-2-3-9(18)12(7)16(19)23-15/h6,8,17H,2-5H2,1H3/t8-,17+/m0/s1
#> 2 InChI=1S/C17H14O7/c1-20-9-6-10-12(8-3-5-22-17(8)23-10)14-11(9)7-2-4-21-15(18)13(7)16(19)24-14/h6,8,17H,2-5H2,1H3/t8-,17+/m0/s1
#> inchikey cas pubchem name
#> 1 WWSYXEZEXMQWHT-WNWIJWBNSA-N 7220-81-7 CID:2724360 Aflatoxin B2
#> 2 WPCVRWVBBXIRMA-WNWIJWBNSA-N 7241-98-7 CID:2724362 Aflatoxin G2
#########
## Using CompDb with the *tidyverse*
##
## Using return.type = "tibble" the result will be returned as a "tibble"
compounds(cdb, return.type = "tibble")
#> # A tibble: 12 × 8
#> formula exactmass smiles inchi inchikey cas pubchem name
#> <chr> <dbl> <chr> <chr> <chr> <chr> <chr> <chr>
#> 1 C10H10O3 178. CC1CC2=C(C(=CC=C2)O)… InCh… KWILGNN… 1739… CID:28… Mell…
#> 2 C25H47NO9 505. CC[C@@H](C)[C@H]([C@… InCh… CTXQVLL… 1498… CID:10… AAL …
#> 3 C17H12O6 312. COC1=C2C3=C(C(=O)CC3… InCh… OQIQSTL… 1162… CID:18… Afla…
#> 4 C17H14O6 314. COC1=C2C3=C(C(=O)CC3… InCh… WWSYXEZ… 7220… CID:27… Afla…
#> 5 C17H12O7 328. COC1=C2C3=C(C(=O)OCC… InCh… XWIYFDM… 1165… CID:27… Afla…
#> 6 C17H14O7 330. COC1=C2C3=C(C(=O)OCC… InCh… WPCVRWV… 7241… CID:27… Afla…
#> 7 C17H12O7 328. COC1=C2C3=C(C(=O)CC3… InCh… MJBWDEQ… 6795… CID:15… Afla…
#> 8 C20H20N2O3 336. CC(=O)C1=C([C@@H]2[C… InCh… SZINUGQ… 1817… CID:54… alph…
#> 9 C15H16O6 292. C[C@]12C[C@@H]([C@H]… InCh… MMHTXEA… 2975… CID:34… Alte…
#> 10 C14H10O5 258. CC1=CC(=CC2=C1C3=CC(… InCh… CEBXXEK… 641-… CID:53… Alte…
#> 11 C15H12O5 272. CC1=CC(=CC2=C1C3=CC(… InCh… LCSDQFN… 2345… CID:53… Alte…
#> 12 C16H16O8 336. C[C@]1([C@@H]([C@H](… InCh… VSMBLBO… 2226… CID:89… Alte…
## Use the CompDb in a dplyr setup
library(dplyr)
#>
#> Attaching package: ‘dplyr’
#> The following objects are masked from ‘package:S4Vectors’:
#>
#> first, intersect, rename, setdiff, setequal, union
#> The following objects are masked from ‘package:BiocGenerics’:
#>
#> combine, intersect, setdiff, setequal, union
#> The following object is masked from ‘package:generics’:
#>
#> explain
#> The following objects are masked from ‘package:stats’:
#>
#> filter, lag
#> The following objects are masked from ‘package:base’:
#>
#> intersect, setdiff, setequal, union
src_cmp <- src_compdb(cdb)
src_cmp
#> src: sqlite 3.51.2 [/__w/_temp/Library/CompoundDb/sql/CompDb.MassBank.sql]
#> tbls: metadata, ms_compound, msms_spectrum, msms_spectrum_peak, synonym
## Get a tbl for the ms_compound table
cmp_tbl <- tbl(src_cmp, "ms_compound")
## Extract the id, name and inchi
cmp_tbl %>% select(compound_id, name, inchi) %>% collect()
#> # A tibble: 70 × 3
#> compound_id name inchi
#> <chr> <chr> <chr>
#> 1 1 Mellein InChI=1S/C10H10O3/c1-6-5-7-3-2-4-8(11)9(7)10(12)13-…
#> 2 2 Mellein InChI=1S/C10H10O3/c1-6-5-7-3-2-4-8(11)9(7)10(12)13-…
#> 3 3 Mellein InChI=1S/C10H10O3/c1-6-5-7-3-2-4-8(11)9(7)10(12)13-…
#> 4 4 Mellein InChI=1S/C10H10O3/c1-6-5-7-3-2-4-8(11)9(7)10(12)13-…
#> 5 5 Mellein InChI=1S/C10H10O3/c1-6-5-7-3-2-4-8(11)9(7)10(12)13-…
#> 6 6 AAL toxin TB InChI=1S/C25H47NO9/c1-4-17(3)24(32)21(35-23(31)13-1…
#> 7 7 AAL toxin TB InChI=1S/C25H47NO9/c1-4-17(3)24(32)21(35-23(31)13-1…
#> 8 8 AAL toxin TB InChI=1S/C25H47NO9/c1-4-17(3)24(32)21(35-23(31)13-1…
#> 9 9 AAL toxin TB InChI=1S/C25H47NO9/c1-4-17(3)24(32)21(35-23(31)13-1…
#> 10 10 AAL toxin TB InChI=1S/C25H47NO9/c1-4-17(3)24(32)21(35-23(31)13-1…
#> # ℹ 60 more rows
########
## Creating an empty CompDb and sequentially adding content
##
## Create an empty CompDb and store the database in a temporary file
cdb <- emptyCompDb(tempfile())
cdb
#> class: CompDb
#> data source: NA
#> version: NA
#> organism: NA
#> compound count: 0
## Define a data.frame with some compounds to add
cmp <- data.frame(
compound_id = c(1, 2),
name = c("Caffeine", "Glucose"),
formula = c("C8H10N4O2", "C6H12O6"),
exactmass = c(194.080375584, 180.063388116))
## We can also add multiple synonyms for each compound
cmp$synonyms <- list(c("Cafeina", "Koffein"), "D Glucose")
cmp
#> compound_id name formula exactmass synonyms
#> 1 1 Caffeine C8H10N4O2 194.0804 Cafeina,....
#> 2 2 Glucose C6H12O6 180.0634 D Glucose
## These compounds can be added to the empty database with insertCompound
cdb <- insertCompound(cdb, compounds = cmp)
compounds(cdb)
#> name inchi inchikey formula exactmass
#> 1 Caffeine <NA> <NA> C8H10N4O2 194.0804
#> 2 Glucose <NA> <NA> C6H12O6 180.0634
## insertCompound would also allow to add additional columns/annotations to
## the database. Below we define a new compound adding an additional column
## hmdb_id
cmp <- data.frame(
compound_id = 3,
name = "Alpha-Lactose",
formula = "C12H22O11",
exactmass = 342.116211546,
hmdb_id = "HMDB0000186")
## To add additional columns we need to set addColumns = TRUE
cdb <- insertCompound(cdb, compounds = cmp, addColumns = TRUE)
cdb
#> class: CompDb
#> data source: NA
#> version: NA
#> organism: NA
#> compound count: 3
compounds(cdb)
#> name inchi inchikey formula exactmass hmdb_id
#> 1 Alpha-Lactose <NA> <NA> C12H22O11 342.1162 HMDB0000186
#> 2 Caffeine <NA> <NA> C8H10N4O2 194.0804 <NA>
#> 3 Glucose <NA> <NA> C6H12O6 180.0634 <NA>
######
## Deleting selected compounds from a database
##
## Compounds can be deleted with the deleteCompound function providing the
## IDs of the compounds that should be deleted. IDs of compounds in the
## database can be retrieved by adding "compound_id" to the columns parameter
## of the compounds function:
compounds(cdb, columns = c("compound_id", "name"))
#> compound_id name
#> 1 1 Caffeine
#> 2 2 Glucose
#> 3 3 Alpha-Lactose
## Compounds can be deleted with the deleteCompound function. Below we delete
## the compounds with the IDs "1" and "3" from the database
cdb <- deleteCompound(cdb, ids = c("1", "3"))
compounds(cdb)
#> name inchi inchikey formula exactmass hmdb_id
#> 1 Glucose <NA> <NA> C6H12O6 180.0634 <NA>
## If also MS2 spectra associated with any of these two compounds an error
## would be thrown. Setting the parameter `recursive = TRUE` in the
## `deleteCompound` call would delete the compounds along with their MS2
## spectra.
#########
## Filtering the database
##
## Get all compounds with an exact mass between 310 and 320
res <- compounds(cdb, filter = ~ exactmass > 310 & exactmass < 320)
res
#> formula exactmass smiles
#> 1 C17H12O6 312.0634 COC1=C2C3=C(C(=O)CC3)C(=O)OC2=C4[C@@H]5C=CO[C@@H]5OC4=C1
#> 2 C17H14O6 314.0790 COC1=C2C3=C(C(=O)CC3)C(=O)OC2=C4[C@@H]5CCO[C@@H]5OC4=C1
#> inchi
#> 1 InChI=1S/C17H12O6/c1-20-10-6-11-14(8-4-5-21-17(8)22-11)15-13(10)7-2-3-9(18)12(7)16(19)23-15/h4-6,8,17H,2-3H2,1H3/t8-,17+/m0/s1
#> 2 InChI=1S/C17H14O6/c1-20-10-6-11-14(8-4-5-21-17(8)22-11)15-13(10)7-2-3-9(18)12(7)16(19)23-15/h6,8,17H,2-5H2,1H3/t8-,17+/m0/s1
#> inchikey cas pubchem name
#> 1 OQIQSTLJSLGHID-WNWIJWBNSA-N 1162-65-8 CID:186907 Aflatoxin B1
#> 2 WWSYXEZEXMQWHT-WNWIJWBNSA-N 7220-81-7 CID:2724360 Aflatoxin B2
## Get all compounds that have an H14 in their formula.
res <- compounds(cdb, filter = FormulaFilter("H14", "contains"))
res
#> formula exactmass smiles
#> 1 C17H14O6 314.0790 COC1=C2C3=C(C(=O)CC3)C(=O)OC2=C4[C@@H]5CCO[C@@H]5OC4=C1
#> 2 C17H14O7 330.0739 COC1=C2C3=C(C(=O)OCC3)C(=O)OC2=C4[C@@H]5CCO[C@@H]5OC4=C1
#> inchi
#> 1 InChI=1S/C17H14O6/c1-20-10-6-11-14(8-4-5-21-17(8)22-11)15-13(10)7-2-3-9(18)12(7)16(19)23-15/h6,8,17H,2-5H2,1H3/t8-,17+/m0/s1
#> 2 InChI=1S/C17H14O7/c1-20-9-6-10-12(8-3-5-22-17(8)23-10)14-11(9)7-2-4-21-15(18)13(7)16(19)24-14/h6,8,17H,2-5H2,1H3/t8-,17+/m0/s1
#> inchikey cas pubchem name
#> 1 WWSYXEZEXMQWHT-WNWIJWBNSA-N 7220-81-7 CID:2724360 Aflatoxin B2
#> 2 WPCVRWVBBXIRMA-WNWIJWBNSA-N 7241-98-7 CID:2724362 Aflatoxin G2
#########
## Using CompDb with the *tidyverse*
##
## Using return.type = "tibble" the result will be returned as a "tibble"
compounds(cdb, return.type = "tibble")
#> # A tibble: 12 × 8
#> formula exactmass smiles inchi inchikey cas pubchem name
#> <chr> <dbl> <chr> <chr> <chr> <chr> <chr> <chr>
#> 1 C10H10O3 178. CC1CC2=C(C(=CC=C2)O)… InCh… KWILGNN… 1739… CID:28… Mell…
#> 2 C25H47NO9 505. CC[C@@H](C)[C@H]([C@… InCh… CTXQVLL… 1498… CID:10… AAL …
#> 3 C17H12O6 312. COC1=C2C3=C(C(=O)CC3… InCh… OQIQSTL… 1162… CID:18… Afla…
#> 4 C17H14O6 314. COC1=C2C3=C(C(=O)CC3… InCh… WWSYXEZ… 7220… CID:27… Afla…
#> 5 C17H12O7 328. COC1=C2C3=C(C(=O)OCC… InCh… XWIYFDM… 1165… CID:27… Afla…
#> 6 C17H14O7 330. COC1=C2C3=C(C(=O)OCC… InCh… WPCVRWV… 7241… CID:27… Afla…
#> 7 C17H12O7 328. COC1=C2C3=C(C(=O)CC3… InCh… MJBWDEQ… 6795… CID:15… Afla…
#> 8 C20H20N2O3 336. CC(=O)C1=C([C@@H]2[C… InCh… SZINUGQ… 1817… CID:54… alph…
#> 9 C15H16O6 292. C[C@]12C[C@@H]([C@H]… InCh… MMHTXEA… 2975… CID:34… Alte…
#> 10 C14H10O5 258. CC1=CC(=CC2=C1C3=CC(… InCh… CEBXXEK… 641-… CID:53… Alte…
#> 11 C15H12O5 272. CC1=CC(=CC2=C1C3=CC(… InCh… LCSDQFN… 2345… CID:53… Alte…
#> 12 C16H16O8 336. C[C@]1([C@@H]([C@H](… InCh… VSMBLBO… 2226… CID:89… Alte…
## Use the CompDb in a dplyr setup
library(dplyr)
#>
#> Attaching package: ‘dplyr’
#> The following objects are masked from ‘package:S4Vectors’:
#>
#> first, intersect, rename, setdiff, setequal, union
#> The following objects are masked from ‘package:BiocGenerics’:
#>
#> combine, intersect, setdiff, setequal, union
#> The following object is masked from ‘package:generics’:
#>
#> explain
#> The following objects are masked from ‘package:stats’:
#>
#> filter, lag
#> The following objects are masked from ‘package:base’:
#>
#> intersect, setdiff, setequal, union
src_cmp <- src_compdb(cdb)
src_cmp
#> src: sqlite 3.51.2 [/__w/_temp/Library/CompoundDb/sql/CompDb.MassBank.sql]
#> tbls: metadata, ms_compound, msms_spectrum, msms_spectrum_peak, synonym
## Get a tbl for the ms_compound table
cmp_tbl <- tbl(src_cmp, "ms_compound")
## Extract the id, name and inchi
cmp_tbl %>% select(compound_id, name, inchi) %>% collect()
#> # A tibble: 70 × 3
#> compound_id name inchi
#> <chr> <chr> <chr>
#> 1 1 Mellein InChI=1S/C10H10O3/c1-6-5-7-3-2-4-8(11)9(7)10(12)13-…
#> 2 2 Mellein InChI=1S/C10H10O3/c1-6-5-7-3-2-4-8(11)9(7)10(12)13-…
#> 3 3 Mellein InChI=1S/C10H10O3/c1-6-5-7-3-2-4-8(11)9(7)10(12)13-…
#> 4 4 Mellein InChI=1S/C10H10O3/c1-6-5-7-3-2-4-8(11)9(7)10(12)13-…
#> 5 5 Mellein InChI=1S/C10H10O3/c1-6-5-7-3-2-4-8(11)9(7)10(12)13-…
#> 6 6 AAL toxin TB InChI=1S/C25H47NO9/c1-4-17(3)24(32)21(35-23(31)13-1…
#> 7 7 AAL toxin TB InChI=1S/C25H47NO9/c1-4-17(3)24(32)21(35-23(31)13-1…
#> 8 8 AAL toxin TB InChI=1S/C25H47NO9/c1-4-17(3)24(32)21(35-23(31)13-1…
#> 9 9 AAL toxin TB InChI=1S/C25H47NO9/c1-4-17(3)24(32)21(35-23(31)13-1…
#> 10 10 AAL toxin TB InChI=1S/C25H47NO9/c1-4-17(3)24(32)21(35-23(31)13-1…
#> # ℹ 60 more rows
########
## Creating an empty CompDb and sequentially adding content
##
## Create an empty CompDb and store the database in a temporary file
cdb <- emptyCompDb(tempfile())
cdb
#> class: CompDb
#> data source: NA
#> version: NA
#> organism: NA
#> compound count: 0
## Define a data.frame with some compounds to add
cmp <- data.frame(
compound_id = c(1, 2),
name = c("Caffeine", "Glucose"),
formula = c("C8H10N4O2", "C6H12O6"),
exactmass = c(194.080375584, 180.063388116))
## We can also add multiple synonyms for each compound
cmp$synonyms <- list(c("Cafeina", "Koffein"), "D Glucose")
cmp
#> compound_id name formula exactmass synonyms
#> 1 1 Caffeine C8H10N4O2 194.0804 Cafeina,....
#> 2 2 Glucose C6H12O6 180.0634 D Glucose
## These compounds can be added to the empty database with insertCompound
cdb <- insertCompound(cdb, compounds = cmp)
compounds(cdb)
#> name inchi inchikey formula exactmass
#> 1 Caffeine <NA> <NA> C8H10N4O2 194.0804
#> 2 Glucose <NA> <NA> C6H12O6 180.0634
## insertCompound would also allow to add additional columns/annotations to
## the database. Below we define a new compound adding an additional column
## hmdb_id
cmp <- data.frame(
compound_id = 3,
name = "Alpha-Lactose",
formula = "C12H22O11",
exactmass = 342.116211546,
hmdb_id = "HMDB0000186")
## To add additional columns we need to set addColumns = TRUE
cdb <- insertCompound(cdb, compounds = cmp, addColumns = TRUE)
cdb
#> class: CompDb
#> data source: NA
#> version: NA
#> organism: NA
#> compound count: 3
compounds(cdb)
#> name inchi inchikey formula exactmass hmdb_id
#> 1 Alpha-Lactose <NA> <NA> C12H22O11 342.1162 HMDB0000186
#> 2 Caffeine <NA> <NA> C8H10N4O2 194.0804 <NA>
#> 3 Glucose <NA> <NA> C6H12O6 180.0634 <NA>
######
## Deleting selected compounds from a database
##
## Compounds can be deleted with the deleteCompound function providing the
## IDs of the compounds that should be deleted. IDs of compounds in the
## database can be retrieved by adding "compound_id" to the columns parameter
## of the compounds function:
compounds(cdb, columns = c("compound_id", "name"))
#> compound_id name
#> 1 1 Caffeine
#> 2 2 Glucose
#> 3 3 Alpha-Lactose
## Compounds can be deleted with the deleteCompound function. Below we delete
## the compounds with the IDs "1" and "3" from the database
cdb <- deleteCompound(cdb, ids = c("1", "3"))
compounds(cdb)
#> name inchi inchikey formula exactmass hmdb_id
#> 1 Glucose <NA> <NA> C6H12O6 180.0634 <NA>
## If also MS2 spectra associated with any of these two compounds an error
## would be thrown. Setting the parameter `recursive = TRUE` in the
## `deleteCompound` call would delete the compounds along with their MS2
## spectra.
